Amerikanska aktiviteter ska driva tillväxt för Integrum
Svenska Integrum har skaffat sig en stark utgångsposition på den stora USA-marknaden, genom att ha utvecklat den enda FDA-godkända metoden för att förankra avancerade proteser direkt till skelettet hos amputerade individer. Resan mot kommersiellt genombrott har redan påbörjats via ett ramavtal med det amerikanska försvaret och bolaget ökar nu intensiteten i marknadsaktiviteterna. BioStock har talat med både Integrums nye försäljningschef James Sheridan och Patrick Treacy, vd på Onkos Surgical, specialister på ortopedi i samband med canceroperationer och en av Integrums viktiga samarbetspartners som bidrar till att etablera tekniken i USA.
I årsrapporten för det brutna räkenskapsåret maj 2019 till april 2020 som släpptes den 2 september redovisade det Mölndalsbaserade medicinteknikbolaget Integrum en årstillväxt om 19 procent. Ett måttligt resultat, menade vd Maria Lopez, i förhållande till bolagets höga ambitioner. Orsaken kunde spåras till uppskjutna operationer relaterat till COVID-19-pandemin, något som direkt påverkat försäljningen men även längden på säljcyklerna i USA, där flera processer inte beräknades få betydande effekt förrän under Q3 och Q4. Bolaget redovisade dock i Q1-rapporten en drygt 80-procentigökning av försäljningen jämfört med Q1 2019. Framförallt kom ökningen från USA och bolaget räknar med att tillväxten kommer att ta ytterligare fart igen när senarelagda operationer kan genomföras.
 Flera år före konkurrenterna
Flera år före konkurrenterna
Integrum ligger flera år före konkurrenterna i utvecklingen och med ett FDA-besked om så kallad premarket approval (PMA) som inväntas i höst, kommer denna inträdesbarriär att bli ännu högre för konkurrenterna. Detta fick BioStock erfara i en tidigare intervju med bolagets styrelseordförande och grundare, professor Rickard Brånemark. Läs mer här.
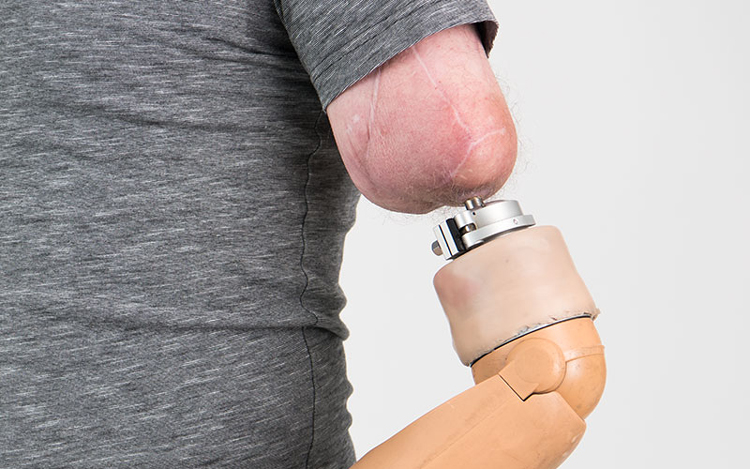
OPRA Implant System
Redan för 58 år sedan upptäckte professor Per-Ingvar Brånemark att titan kan växa samman med skelettvävnad. Tekniken, osseointegration, har sedan dess framgångsrikt använts för att förankra tandimplantat i käken. År 1998 grundades Integrum av uppfinnaren tillsammans med sonen Rickard Brånemark och idag har konceptet expanderat bortom tandvård till att omfatta avancerade proteser för amputerade.
Med OPRA Implant System kan proteser förankras direkt till skelettet, istället för den konventionella tekniken att förankra protesen i en hylsa. Implantatsystemet är därtill det enda systemet som kan uppgraderas med implanterade elektroder och artificiell intelligens vilket ger patienter naturlig tankestyrd kontroll över sina proteser, samtidigt som de kan förmedla sensorisk återkoppling, det vill säga en sorts konstgjord känsel som gör att protesen inte upplevs som främmande utan en naturlig del av kroppen.
Integrum har även tagit fram Neuromotus, en teknik baserad på Augmented Reality och maskininlärning som används för att minska fantomsmärta efter en amputation. Bolaget har genomfört kliniska studier hos amputerade och där ingen annan terapi har hjälpt och dessa data visar att fantomsmärtan kan reduceras upp till 50 procent.
Flera samarbeten i USA
Integrum satsar nu hårt mot att nå ut på den viktiga amerikanska marknaden. Under kalenderåret 2020väntas som nämnts ovan ett besked från FDA rörande bolagets ansökan om s.k. Premarket Approval (PMA).Ett godkännande skulle dels göra det enklare att få behandlingen godkänd för ersättning eller reimbursementav fler försäkringsbolag, samtidigt det skapar underlag för ett godkännande inom ramarna för Medicare, försäkringssystemet för pensionerade i USA.
I oktober 2017 fick Integrum en första order av det amerikanska försvarsdepartementet via militärsjukhuset Walter Reed National Military Medical Center gällande 20 lårbensoperationer av amputerade amerikanska soldater. Ett halvår senare tecknades ett ramavtal värt 60 Mkr, vilket kan ses som ett ypperligt kvitto på Integrums behandlingskoncept.
Utöver detta har Integrum etablerat forskningssamarbeten med välrenommerade institutioner såsom MIT och Harvard Medical School samt ledande amerikanska sjukhus som Johns Hopkins och ovan nämnda Walter Reed Army Medical Center.
Distributionspartner bidrar till marknadspenetration
I början av 2020 ingick Integrum även ett flerårigt samarbetsavtal med amerikanska Onkos Surgical med fokus på den amerikanska marknaden, där parterna gemensamt skall verka för att snabbare etablera OPRA implantatsystem på viktiga kirurgiska centra i USA. Onkos Surgical har genom sitt starka lokala nätverk direktkontakt med ett stort antal kliniker och kirurger och man har även lång erfarenhet av samarbete med amerikanska försäkringsbolag i reimbursementfrågor.
På pappret gör detta Onkos Surgical till en idealisk partner för ett bolag i Integrums situation, där en spjutspetsteknologi skall introduceras brett på en etablerad marknad där upparbetade kontaktytor är av största betydelse. Så sent som på måndagen levererades också ett ytterligare ett konkret kvitto på att samarbetet inletts väl, när Integrum kunde presentera ett prestigeladdat avtal – insålt via Onkos Surgical – med Keck Medical Center vid University of Southern California. (Läs mer om avtalet här.)
Integrums försljningschef och Onkos Surgicals vd kommenterar
BioStock har fått möjlighet att prata med både Integrums nytillträdde försäljningschef James Sheridan och med Patrick Treacy, vd på Onkos Surgical, Integrums amerikanska samarbetspartner om pågående marknadsaktiviteter, hur bolagets produkter tagits emot och de viktigaste parametrarna för att driva fortsatt tillväxt.

James Sheridan, Head of Sales and Marketing EMEA
»An essential facet of Integrum’s strategy is to increase collaboration with the key centres in the major markets.« – James Sheridan, Head of Sales and Marketing EMEA Integrum
James, could you start by telling us a bit about your professional background and experiences?
– I have been working within Medical Devices for over twenty years; primarily within Dental Implantology. My career thus far has taken me to Japan, Australia, and then back to Sweden and the Nordic Markets. In Japan, I worked within AstraZeneca, in close collaboration with distributors in bringing a new dental implant system to the Japanese market. Within three years we had built a solid market position as one of the leading premium brands.
– In Australia, my initial responsibility as Business Unit Manager Dental, was to launch a new dental implant system and build a local Sales and Marketing Team. After two years, and a period of very rapid growth, I became Managing Director of Astra Tech Pty. LTD, and assumed responsibility for the Healthcare/Hospital business as well. After seven years, the dental implant business had achieved a leading market position, and the local organisation had grown to a team of 35 people.
– Most recently, as Managing Director, Dentsply Sirona Implants Nordic, I was responsible for the Sales and Marketing of dental implants and related surgical and prosthetic products in the Nordic markets.
What attracted you to the role as Head of Sales and Marketing at Integrum?
– I have always enjoyed working with scientifically well documented products which improve patient quality of life. I strongly feel that Integrum’s OPRA and E-Opra technology offer a significant improvement to patient quality of life. Professionally speaking, the opportunity to be a part of leading and driving Integrum’s commercial development is a once in a lifetime opportunity.
In your role at Integrum you will be responsible for sales and marketing for the EMEA area (Europe, Middle East and Africa), i.e. areas outside the US where Integrum already has a sales organisation. How is Integrum currently positioned on these markets?
– Through its international network of world renowned scientists and clinicians, Integrum has built relationships, and initiated commercial activities with, key clinics and potential Centres of Excellence in many of the most important markets outside of the United States. These, and future potential centres, are currently being served by the dedicated team in Gothenburg.
What is Integrum’s strategy to expand in these markets and what are the most important factors for a successful expansion?
– An essential facet of Integrum’s strategy is to increase collaboration with the key centres in the major markets. Unfortunately, most patients – and many clinicians – are not yet aware of the scientifically documented benefits of the OPRA Implant System, as compared to other bone anchored, or traditional socket type prostheses.
– Consequently, we will work jointly with the key centres and patient groups in these markets in order to increase public awareness. Without this awareness, it is impossible for patients or care givers to make informed decisions regarding the best solution for each individual patient.
To what extent has the Covid-19 pandemic impacted how you conduct sales?
– Starting early this year, sales activity was greatly impacted by the pandemic. Access to hospitals and clinicians in many countries has been difficult to say the least. Moreover, many hospital units have completely shut down all forms of surgical treatment, reallocated their resources, and placed their focus on the treatment of patients with covid-19.
– At present, restriction levels in many countries are starting to ease, which means that treatment-, and thereby sales-, activity levels will increase steadily throughout the autumn of 2020. For those markets which remain more restrictive, we will continue to support our customers and prospects remotely, and where possible, further augment these activities with physical training and support.
Can you tell us something about Integrum’s near-term goals in term of expanding on relevant markets, outside the US?
– In the near future, Integrum will place its focus on expanding in those markets where potential treatment volumes are high, surgical/prosthetic/rehabilitative skills are at a high level, and where some centers are already trained and working with the OPRA system. These are markets such as Australia, Austria, Belgium, France, and Germany.

Patrick Treacy, CEO Onkos Surgical
»Integrum has a tremendous competitive advantage, they have the only FDA-approved device and as such, their device is the only legally marketable device. So Integrum have a very strong regulatory position in comparison to competitors.« – Patrick Treacy, CEO Onkos Surgical
Patrick, in January of this year Onkos Surgical partnered with Integrum for a multi-year collaboration. How did this collaboration come about and what attracted you to Integrum as a partner?
– I had known about Integrum and Rickard Brånemark, who of course has a very strong reputation and is well-known for his expertise, for several years when I was introduced to him in 2015. Onkos Surgical have always wanted to have an osseointegration product and Integrum’s product is probably the best one with the most clinical data on the market. Instead of Onkos starting from scratch developing our own product we felt that a collaboration with Integrum would be perfect and in January of this year we had settled on the right set-up for the collaboration.
Can you tell us a bit more about how the collaboration works?
– It was very important for Integrum to have the end market presence in the US, so Onkos is contracted as a distributor of OPRA in certain accounts, primarily focusing on tumour- and bone cancer patients. In short, we have access to a set of customers that have a need for Integrum’s product in a space where Integrum did not have a presence, meaning that Onkos could bring the product and the customers together.
Onkos Surgical has an extensive network of US hospitals – how has Integrum’s product been received by hospitals in the US?
– If we look at how our surgeon customers have received the product, these customers have wanted access to a product like OPRA for some time, so the product has been very well received by surgeons.
–When it comes to hospitals, the most common questions that we get are concerning reimbursement and coverage, i.e. who is going to pay for the product. Onkos Surgical has extensive experience in helping hospitals to work with the insurance companies when it comes to getting devices like OPRA paid for. We have made good progress here and have gotten the product through at major academic medical centres such as Emory University, in Georgia and, most recently, University of Southern California (USC) and we have additional universities in pipeline.
What is the competitive landscape like and how do you expect Integrum to position itself on the US market?
– Integrum has a tremendous competitive advantage, they have the only FDA-approved device and as such, their device is the only legally marketable device. So Integrum have a very strong regulatory position in comparison to competitors.
During the second quarter of 2020 Integrum has seen continued growth, largely driven by activities on the US market. What is Onkos strategy for the US market?
– We will continue to bring the product to the clients we are accounted for and plan to continue to grow. We of course also hope to expand our account list. A very important factor is that we have strong relationships with leading cancer centres and major academic centres, meaning that we have easy access to the leadership and can introduce OPRA to them.
When do you expect that the first OPRA surgery will be carried out at one of ‘your’ centres?
– Hopefully within the coming weeks. USC, West Virginia University and Emory University are all ready and have patients lined up, so where the first OPRA surgery is performed depends on when the first patient is scheduled.
What are the most important factors for ensuring success on the US market for Integrum?
– I would say that one very important factor is their investment in the team in the US. It is vital to train the team and to build expertise so that the expansion of the company is not reliant on Rickard’s knowledge and expertise. So, I would point to the ability to force-multiply, train the team and being able to respond to the initial cases quickly as important factors for Integrum’s ability to grow.
So, a key factor is educating the medical community about the OPRA-system and how to use it. Can you tell us a bit about how you are doing this?
– Fortunately for us, with regards to the Covid-19 pandemic, we were already using a digital work model. We hold individual one-on-one case planning sessions with surgeons online and we have also been holding webinars with surgeons. We have, to give an example, worked with targeted groups of surgeons in less formal settings so that they can feel comfortable asking questions. Also, recently we had a surgeon being trained online by Dr Brånemark in preparation for the first case. I must say that Dr Brånemark has been fantastic when it comes to the training.
In conclusion, what do you hope for with regards to Onkos Surgical’s collaboration with Integrum?
– I of course look forward very much to the first OPRA surgeries being performed! We will also continuing training and hope to expand our target, so far Onkos has brought in two very prestigious institutions in a short period of time and I hope this trend will continue. In my opinion, Integrum has a unique, high-quality product that we can offer our customers so my hope is that we will continue to grow the customer base for Integrum.
Läs även: Integrum inväntar viktig milstolpe på väg mot kommersiellt genombrott (publicerat 20 augusti 2020)
Innehållet i BioStocks nyheter och analyser är oberoende men BioStocks verksamhet är i viss mån finansierad av bolag i branschen. Detta inlägg avser ett bolag som BioStock erhållit finansiering från.
 Flera år före konkurrenterna
Flera år före konkurrenterna